A message of Hope
This document has been created to summarise the message of hope for Syngap families, written for a non-scientific audience (laymen).
There are seven sections that cover:
- The new medical era of knowledge, technology and funding, making Precision Medicine a realistic proposition for rare diseases;
- Not only does Precision Medicine offer promising options for Syngap, viable treatments and cures are now approved for many conditions;
- Research in animal models show the impacts of Syngap can be reversed when normal syngap production is restored, giving hope to families of Syngap patients of all ages;
- We are in a new era of drug development for epilepsy specifically, Syngap is a genetic epilepsy;
- The Syngap community is mobilised;
- The Syngap research roadmap to a cure… research and projects.
- Syngap continues to attract astute researchers.
You can find this page in a PDF format by clicking HERE
Precision Medicine – a new medical era of knowledge, technology and funding
There has never been a better time to rely on science, medical discoveries, gene therapy or precision medicine for an answer. We are collectively at the precipice of a new medical era.
The Syngap community is lucky to have a talented, knowledgeable and relentless group of Syngap researchers currently working on Syngap; understanding, treating and creating cures for Syngap. Some of these researchers were involved in Syngap from the beginning. For example; Richard Huganir and Mary Kennedy who co-discovered Syngap1 through identification of the major protein products of the gene in 1998, Dr Jacques Michaud who discovered the first patients with pathogenic Syngap mutations in 2009, Gavin Rumbaugh and James Clement who delved further into the neurological consequences of Syngap1 haploinsufficiency since 2011 and Professor Scheffer who published the first paper on the natural history of a large cohort of Syngap patients in 2019. These giants of the industry have led the way for hundreds of Clinicians, scientists and researchers who now dedicate their time to Syngap research, collectively publishing 231 scientific papers, establishing more than 50 Syngap projects across more than 12 countries.
Syngap is a rare neurological condition, genetic epilepsy and a Developmental Epileptic Encephalopathy (DEE). While epilepsy has historically been a very successful field for drug development, with the identification of genes causing epilepsy (927), genetics has moved the epilepsy treatment field from treating symptoms to treating genes.
A focus on the cause of the symptoms (the gene) has heralded in a new era of genetic based epilepsy drug development (Ana Mignorance), we’re moving beyond just treating symptoms to tackle the cause. Precision Medicine is now delivering viable treatments and cures for many conditions including genetic epilepsies (see appendix 1 & 2).
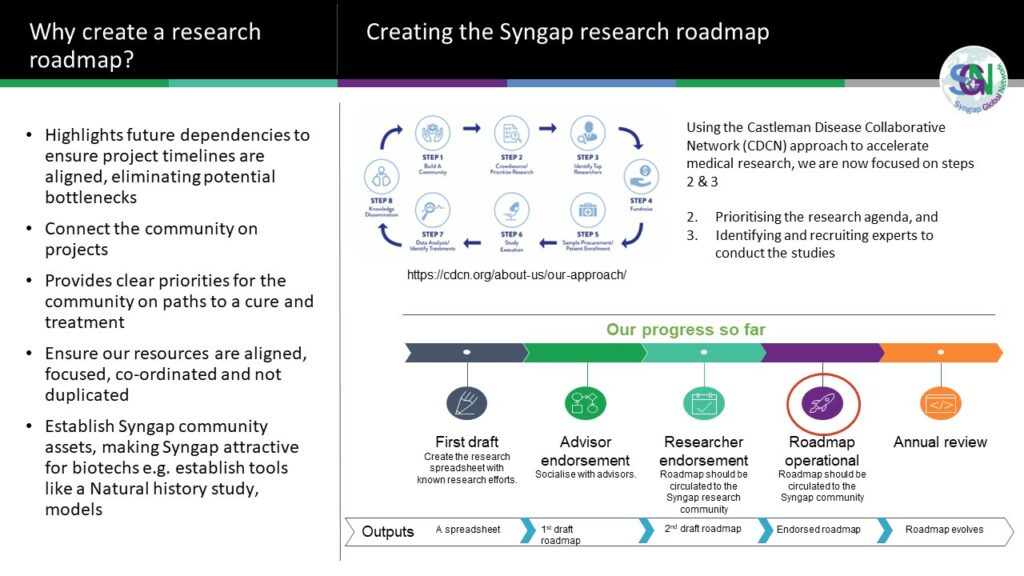
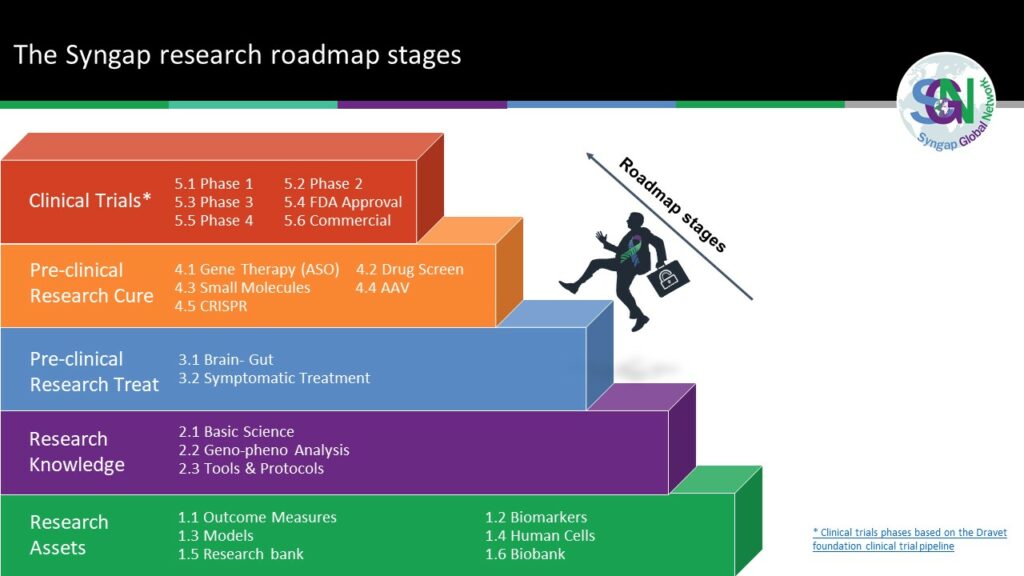
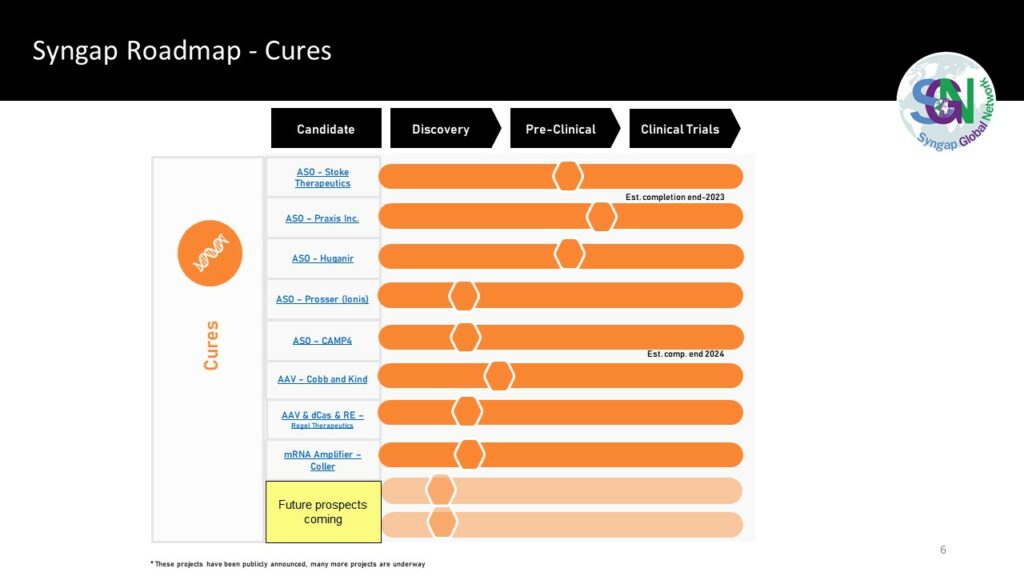
In 2020, the Syngap Global Network (SGN) created the first Syngap research roadmap. Now there is at least one project underway in each of the five areas for Curing Syngap; Gene Therapies (ASO), Drug Screen, Small Molecules, AAV and CRISPR. Gene specific therapies – the next therapeutic milestone in neurology (biomedcentral.com).
Across the Syngap Global Network, multiple projects are established and many more projects in the planning stages. See Section 2 – the Syngap roadmap to a cure for detail.
Precision Medicine is now delivering viable treatments and cures
Gene Therapy (ASO)
Spinraza is the brand name for the gene therapy drug (Nusinersen), developed to treat Spinal Muscular Atrophy (SMA). Spinraza is a rare disease success story, using an ASO gene therapy technique. The FDA approved Spinraza following the clinical trial that showed a significant reduction in the risk of death or permanent ventilation for trial participants who usually have a life expectancy of 2 years.
For more examples of gene therapy success stories, refer to Appendix 1.
Refer to the overview of SYNGAP1 Treatments under development (July 2021). On the Syngap roadmap of hope, there are now four biotechs who have announced they are working on an ASO for Syngap.
- Praxis Precision Medicines, on April 27, 2022 Praxis announced they are working on an ASO for Syngap.
- Stoke Therapeutics have a great track record with an ASO for Dravet syndrome and are now working with Arcadia on the Syngap ASO. Stoke’s TANGO technology has positive results for Syngap
- Dr. Richard Huganir’s lab demonstrated Syngap increase after targeting a natural antisense transcript (Syngap1-AS) and received a SFARI grant to continue the work.
- Ben Prosser of the University of Pennsylvania was awarded $25m to progress an ASO therapy for Syngap and one other genetic epilepsy.
Drug Screen, Gavin Rumbaugh and his team have created an extensive drug screening platform for Syngap. The team plan to use the platform to run through more than 100,000 different experimental compounds in 2021. “That’s going to be really exciting for drug discovery efforts for SYNGAP1.”
AAV, Cobb & Kind work on AAVs at the University of Edinburgh has been ongoing for the last few years, a grant for Syngap was officially provided in 2023. The latest entrant into this space is Regel Therapeutics who announced in 2023 that they will be focusing on Syngap, leveraging their T3 platform for the treatment of genetic disorders to deliver three levels of specificity aimed at minimizing potential off-target effects while increasing treatment efficiency.
CRISPR. CRISPR is now a modern term… it is incredible to think that its potential for editing genes was published just ten years ago. In a mere decade, the groundbreaking discovery has been successfully developed to the point that many clinical trials are underway. (www.oligotherapeutics.org). Molly Reilly is a post doctorate fellow appointed to the Heller Lab at the University of Pennsylvania, she is using CRISPR tools to modify Syngap.
Research in animal models shows the impacts of Syngap can be reversed
Research in animal models shows the impacts of Syngap can be reversed when normal syngap production is restored, giving hope to families of Syngap patients of all ages.
Re-expression of Syngap protein in adulthood improves measures of brain function and behaviour, showing improvements in epileptic activity (EEG), seizures and memory.

New era of epilepsy drug development
Ana Mignorance details the new era of epilepsy drug development in her talk for the Cleveland Clinic in 2020. “Companies with genetic approaches like the rare epilepsies because clinical trials are easier.” In a 2022 presentation in Utrecht, Ana goes on to say that “some rare diseases are the stepping stone, serving as the “gateway indication” to de- risk a compound before approaching the larger disease. These are often genetic diseases.
Gavin Rumbaugh often talks about this concept. He believes upregulating Syngap will have broader implications for common disorders like stroke, neurodegenerative disorders. Attracting broader interest from biotechs.
Biotechs are now incentivised to research and invest in rare disease with the introduction of the orphan drug act in the US in 1987 and the emergence of multiple government funding programs (like URGenT for ultra-rare gene based therapy). These make it compelling and lucrative for biotechnology companies to invest in rare disease research, including Syngap.
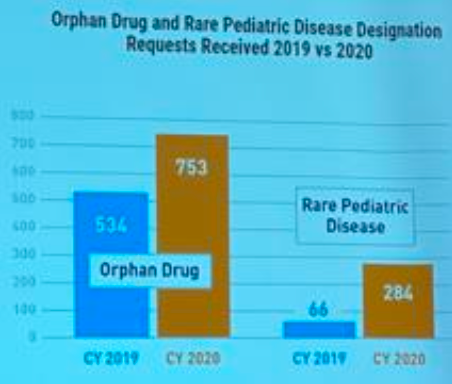
The orphan drug act and funding mechanisms are making a massive difference to the pipeline of orphan drugs being approved by the FDA. It is encouraging to see the number of orphan and rare paediatric drugs approved by the FDA increase by 41% and 330% respectively from 2019 to 2020 (Kathie Bishop, FDA 2020).
The Syngap community is mobilised.
We have established a lot of the required elements to enable and accelerate research.
The Syngap community has…
a) Built a pipeline (see section 2. Syngap roadmap to a cure)
• 50+ projects in 12+ countries.
b) Become trial ready
• Syngap Global Network (SGN) comprises 17 Syngap foundations from around the world, a quarterly census is performed by SGN and Syngap Research Fund US. There are 1,215 confirmed Syngap patients globally.
c) Established and promoted registries (data and biobanks)
• Created the Syngap rare base biobank repository
• 3 x Natural History Studies
• 1 x AI driven databank (Ciitizen)
• 1 x european patient registry under development (as part of the EURAS project)
d) Invested in science, tools and collaborative partnerships
• Pursuing 4 biomarker projects
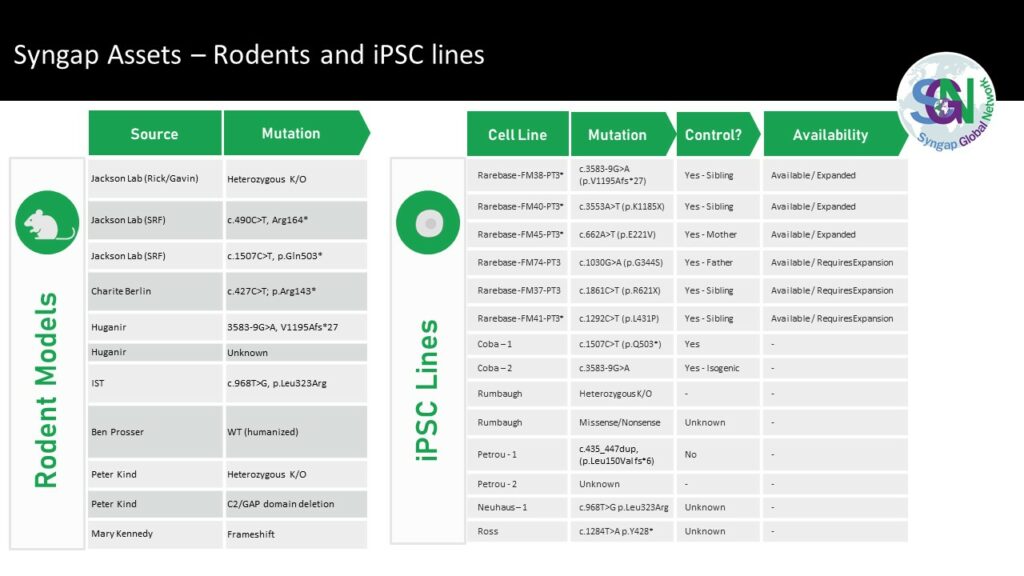
• Created 7 different model types with 47+ available cell lines and 13+ animal models.
• To leverage learnings and share resources, Syngap Foundations collaborate with multiple rare disease consortiums, e.g. Combined Brain, Genetic Epilepsy Team Australia, Genetic Alliance UK, Global Genes, The Personalised Medicine Coalition, The Epilepsy Foundation, Epilepsy leadership council, Simon’s Searchlight, Rare Disease UK, Rare Epilepsy Network, Cambridge rare disease network, RareX and many more.
e) Fundraised and invested in Syngap research
• In 2022 alone, global Syngap foundations provided $3m of grants to Syngap research
• The NIH (American medical funding body) funded $5.6m for Syngap research in 2022. Vicky Whitmore from the NIH (AES 2022) said that “seed funding from the community had generated the increased investment from the NIH”.
• Simons Searchlight gave five Syngap grants, totaling more than $1m.
• In 2022, $10m was channeled into Syngap research efforts. This doesn’t include the significant (tens of millions USD) investment in Syngap from the biotechnology companies who are pursuing therapies for Syngap.
• End of 2022, the EURAS Consortium (initiated by SYNGAP Elternhilfe Germany) received the positive news about a HORIZON Europe funding worth more than 8 Mio € for their research on SYNGAP1 and 3 other disorders
Syngap research roadmap to a cure... research assets and projects
Syngap continues to attract astute researchers for these reasons
a) Surprisingly common – Syngap is one of the most common single-gene determinants of autism (Michaud research). The current patient population meaningfully under-represents totals;
b) Targetable mutations – Most mutations are targetable through ASOs and Syngap has favourable binding properties;
c) Haploinsufficient – It is a haploinsufficiency so Syngap insights may help identify insights on other
haploinsufficiencies (or insights from other haploinsufficiencies could aid Syngap research i.e., Dravet);
d) Cracking Syngap will have impacts beyond the Syngap community – Syngap absorption and production is related to schizophrenia, Alzheimer’s, dementia and many other neurological conditions, insights here could help broader populations (There is no clinical data to suggest that haploinsufficiency of the Syngap1 protein will result in schizophrenia);
e) Established infrastructure – The infrastructure for successful clinical trials either exists or are being established for Syngap, biomarkers and natural history study including access to assets, a network of engaged clinicians and patients;
f) We are a well-organized & passionate community who have created the assets to make it simpler for researchers to work on Syngap.
Co-ordinated by:

Developed and maintained by: Danielle Williams, Pavel Gerovich and Syngap PhD student Montanna Waters
Appendix 1
Precision medicine precedents
• SMA, there are now several approved treatments for SMA.
• Dravet, “it was incredible… we went from seizures and SUDEP (sudden death from epilepsy) in 70% of the mice to almost none.” Isom said.
• Batten Disease, enzyme replacement therapy
• RETT syndrome, first treatment approved by the FDA in 2023 after years of clinical trials.
• Praxis Precision Medicines PRAX-222 (pre-clinical) for SCN2A
• Stoke Therapeutics‘ MONARCH study (clinical phase 1/2A) for Dravet Syndrome
• GeneTX and Ultragenyx GTX-102 trial (phase 1/2) for Angelman Syndrome
• Rutgers have published a new tool to identify the best existing treatment option for each epilepsy
• New treatments are continuously being approved. For example; efficacy of adjunctive cenobamate (YKP3089) for the treatment of focal epilepsy.
• Yale physicians are using an implantable device to personalize therapy for those with certain types of epilepsy.
• The US Food and Drug Administration approved purified cannabidiol extract for two genetic epilepsies
Appendix 2
Valuable references
• https://m.facebook.com/story.php?story_fbid=pfbid02UeLCkmjyGwGLa6tkdhHETCvbrkWozmuzo3oUxvYg837bM6kk hHmGVvvX1DJQMygal&id=100068850130236 – „SYNGAP10“ weekly 10 minutes update on research progress by SRF CEO Mike Graglia. Numerous webinars that informed parents about new research approaches and treatment options
• Jeff Coller (mRNA): https://curesyngap1.org/webinars/68-harnessing-messenger-rna-metabolism-for- the-development-of-precision-gene-therapy-syngap1
• Michael Courtney (SYNGAP1 pathways and missense research) https://curesyngap1.org/webinars/investigating-the-functional-single-cell-biology-of-syngap1- pathways
• Gavin Rumbaugh (drug screening, measuring the level of SYNGAP1 protein, also working on a Leon and friends project). https://curesyngap1.org/webinars/dr-gavin-rumbaugh-phd-scripps-compounds-that-regulate- syngap1-protein-expression
• Rick Huganir (Rescue of Syngap expression in SYNGAP1 Syndrome: Antisense Oligonucleotides (ASOs), small molecules, and viral genetic rescue) https://curesyngap1.org/webinars/rescue-of-syngap-expression- in-syngap1-syndrome-antisense-oligonucleotides-asos-small-molecules-and-viral-genetic-rescue-dr-huganir-hopkins
• Gavin Rumbaugh Webinar on Syngap1 Research Roadmap 2020 https://curesyngap1.org/webinars/syngap1-research-roadmap-dr-rumbaugh-scripps
Appendix 3
Links for cures and treatments slide
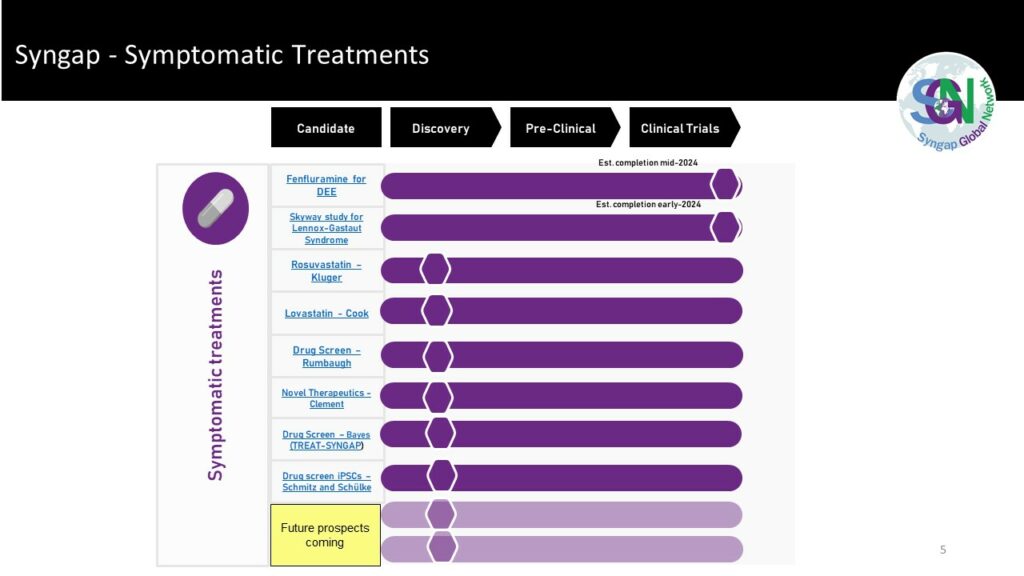
Symptomatic Treatments Links:
Skyway study for Lennox-Gastaut Syndrome
Drug Screen – Bayes (TREAT-SNGAP)
Drug screen iPSCs – Schmitz and Schülke
Cures Links: